Life science companies face big challenges when trying to sell their products. They have to figure out how much their product is worth, get ready to negotiate, and organize a lot of data and documents to show their potential partners. This helps partners process validation data on time, and thus make decisions without any hassle.
A virtual data room is a great solution for this. It keeps information safe and offers many helpful features. Not only does it ensure the security of information, but it also provides an array of beneficial features. Consequently, it simplifies processes, mitigates risks, and enhances the likelihood of successful deals. Let’s delve into the specific ways in which a data room facilitates these outcomes.
What is a virtual data room for life sciences?
A virtual data room (VDR) tailored for life sciences organizations serves as a secure digital repository designed specifically to manage and share sensitive information related to the commercialization of assets in the life sciences sector.
It provides a centralized platform where companies can organize and store data, including scientific research, clinical trial results, regulatory filings, intellectual property documents, and financial information.
Unlike traditional physical data rooms or generic online file-sharing platforms, a VDR for life sciences offers advanced security features such as encryption, access controls, and audit trails to safeguard confidential data and ensure compliance with industry regulations like HIPAA and GDPR.

Moreover, a VDR streamlines collaboration and due diligence processes by enabling authorized parties, including potential partners, investors, and regulatory agencies, to access relevant documents remotely from anywhere in the world.
Through intuitive interfaces and customizable workflows, life science companies can efficiently present information, track user activity, and facilitate seamless communication among stakeholders.
What are the benefits of using virtual data rooms for life sciences?
Data rooms offer powerful functionality tailored specifically to the needs of biotech companies. Let’s take a closer look at key features across data protection, information management, analytics, deal monitoring, and other functionalities of a data room..
1. Data protection
Expect top-tier data security as the foremost priority of each virtual data room. These platforms ensure the confidentiality and integrity of sensitive clinical trial data, safeguarding against breaches and other potential threats.
With SOC 2 Certification, ISO 27001 Certification, SSAE 16 Certification, and robust data center security measures, a data room guarantees the utmost protection for valuable information.
2. Data management
Unlike other software solutions, virtual data rooms make it easy to organize essential data in a secure centralized hub for sensitive data protection related to clinical trials, research findings, regulatory filings, and intellectual property. This way, it ensures accessibility and ease of use for professionals at every level, from clinical researchers to CEOs.
3. Deal progress monitoring
Using a virtual data room also significantly simplifies the monitoring of deal or project progress. Through a unified dashboard accessible to all stakeholders regardless of their location, progress can be tracked and workflow efficiently managed.
4. Analytics
Each data room provides advanced analytics that monitors document engagement, tracking time spent and user activity. This ensures effective communication and a clear track record of all interactions within the data room.
5. Cost optimization
In the life sciences sector, a data room plays a crucial role in cost optimization through advanced analytics, using which firms can monitor user engagement. This ability enables life science companies to allocate resources more efficiently, thus identifying potential areas for cost savings.
Furthermore, by facilitating remote collaboration and eliminating the need for physical meetings, virtual data rooms reduce overhead costs associated with travel and onsite meetings.
6. Information sharing in clinical trials
Last but not least, each virtual data room offers tailored information management solutions for the life sciences sector. Through advanced features such as granular access controls, multi-factor authentication, encryption, and audit trails, each virtual data room ensures compliance with industry regulations and safeguards confidential information.
Additionally, these platforms streamline collaboration by enabling real-time access to documents from anywhere in the world, facilitating seamless communication among researchers, sponsors, regulators, and other stakeholders.
7. Scalability
Life science projects vary in scope and data volume. The software provides scalable storage solutions that can be adjusted based on project demands. This flexibility ensures that life science professionals can efficiently manage their data, keeping costs in check.
8. Conflict management
Data rooms maintain a comprehensive and transparent record of all interactions. So, in cases where a conflict of interest is suspected, the logs serve as a valuable resource for tracing the issue back to its root, thereby enabling timely and effective intervention.
9. Streamlined acquisitions
Secure communication and data sharing in virtual data rooms ensure that the acquiring firm and the target are fully informed about each other’s activities and have all the necessary information for a smooth transition.
Top 3 data rooms
Overall rating:
4.9/5
Excellent

Overall rating:
4.7/5
Excellent

Overall rating:
4.6/5
Good
How do LS and biotech companies use data rooms?
Life science and biotech companies leverage virtual data rooms across various facets of their operations. Here are a few of the most common use cases:
- Clinical trials: For clinical research firms and medical device companies, maintaining the confidentiality of patient data is paramount. Virtual data rooms offer a secure and streamlined solution for sharing sensitive medical information with patients, researchers, and regulatory authorities involved in biomedical experiments.
- Healthcare M&A due diligence: Virtual data rooms facilitate every stage of due diligence during healthcare M&A transactions. By centralizing sensitive documents in a secure repository, companies can ensure thorough examination while preventing unauthorized access or distribution of confidential information.
- Biotech fundraising: Efficiently presenting documents is essential for successful biotech fundraising. Virtual data rooms offer a user-friendly platform for sharing detailed documentation, including DICOM files for medical imaging. Pre-established data room indexes streamline document retrieval, saving investors valuable time.
- IP management: Managing intellectual property portfolios is critical for science-based innovators. Virtual data rooms provide a secure environment for safeguarding and maximizing the value of IP assets, helping companies protect against legal violations and maintain control over their innovations.
- IP licensing: During IP licensing deals, virtual data rooms ensure careful handling of sensitive information. Access controls restrict document access to approved parties only, maintaining confidentiality throughout the licensing process.
- Biotech IPO: Preparing for a biotech IPO involves meticulous management of financial and legal documents. Virtual data rooms streamline this process by providing a centralized repository for critical records, including articles of organization and financial statements. These documents can be securely accessed and reviewed by relevant parties during due diligence.
How to organize virtual data rooms for biopharma partnering
While the structure of every virtual data room provider may vary according to individual preferences, several key components are crucial for biotechnology firms. Commonly, these include:
1. Corporate overview
The corporate overview folder contains essential information about the firm, including its focus, teams, advisory boards, and corporate structure. It serves as a valuable resource during acquisitions, facilitating a smooth transition and providing contact details for necessary interventions.
Folder structure example: 📁Corporate Overview ➡️ 🗂️Company Profile, Team Bios, Advisory Board, and Corporate Structure ➡️ 📄Company presentations, executive bios, organizational charts, contact lists, etc.
Note!
📁 Folder
🗂️ Subfolder
📄Documents
2. Investment overview
In the investment overview folder, information on each stage of the due diligence process is prepared for accessibility based on confidentiality requirements. This ensures both security and ease of access to critical information.
Folder structure example: 📁Investment Overview ➡️ 🗂️Preliminary Due Diligence, Confidential Due Diligence, and Final Due Diligence ➡️ 📄Investment teasers, financial models, due diligence checklists, legal agreements, etc.
3. Commercial strategy
The commercial strategy folder contains detailed insights into the firm’s commercial strategy, including market research, competitive analysis, and commercial forecasts, valuable for corporate planning and acquisitions.
Folder structure example: 📁Commercial Strategy ➡️ 🗂️Market Research, Competitive Analysis, and Commercial Forecasts ➡️ 📄Market reports, competitor profiles, SWOT analysis, revenue projections, etc.
4. Preclinical program
Encompassing completed, ongoing, and planned studies, the preclinical program folder holds vital information and relevant data central to the firm’s focus. It includes study descriptions, timing, status, completed reports, and published manuscripts.
Folder structure example: 📁Preclinic Program ➡️ 🗂️Completed Studies, Ongoing Studies, and Planned Studies ➡️ 📄Study protocols, investigator brochures, study reports, etc.
5. Clinical program
Similar to the previous folder, the clinical program folder includes program interviews and completed studies, detailing descriptions, timing, and status. The distinction lies in the progression to clinical trials, indicating studies that have met approval requirements.
Folder structure example: 📁Clinic Program ➡️ 🗂️Ongoing Studies, Completed Studies, and Upcoming Studies ➡️ 📄Study protocols, informed consent forms, clinical study reports, etc.
6. Intellectual property
Containing patent estate details, granted and pending patents, and other legal documents, this folder safeguards intellectual property rights crucial for the firm’s success.
Folder structure example: 📁Intellectual Property ➡️ 🗂️Granted Patents and Pending Patents ➡️ 📄Patent applications, granted patents, patent search reports, licensing agreements, etc.
7. Regulatory requirements
A regulatory requirements folder must be easily available for reference during each clinical trial phase. The folder should contain the investigator’s brochure, the investigational new drug (IND) application, a log of all regulatory discussions, and a repository of relevant historical regulatory documents.
Folder structure example: 📁Regulatory requirements ➡️ 🗂️Preclinical, Phase I/II, and Phase III ➡️ 📄Investigator brochures, IND applications, regulatory correspondence, FDA meeting minutes, etc.
7. Manufacturing
A comprehensive manufacturing folder includes essential components such as facility overview, contracts, policies and procedures, process and process validation data, inspection documentation, and certificates.
Folder structure example: 📁Manufacturing ➡️ 🗂️Facility Overview, Quality Assurance, and Regulatory Compliance ➡️ 📄Facility layouts, SOPs, batch records, inspection reports, certificates of compliance, etc.
What are the top VDRs for life sciences for biotech?
When it comes to virtual data rooms (VDRs) tailored for life sciences and biotech industries, selecting the right provider is crucial. These industries demand robust security measures, advanced user permissions, and efficient collaboration tools to safeguard sensitive data and streamline processes.
Here are some of the best virtual data rooms renowned for their tailored solutions for biotech companies:
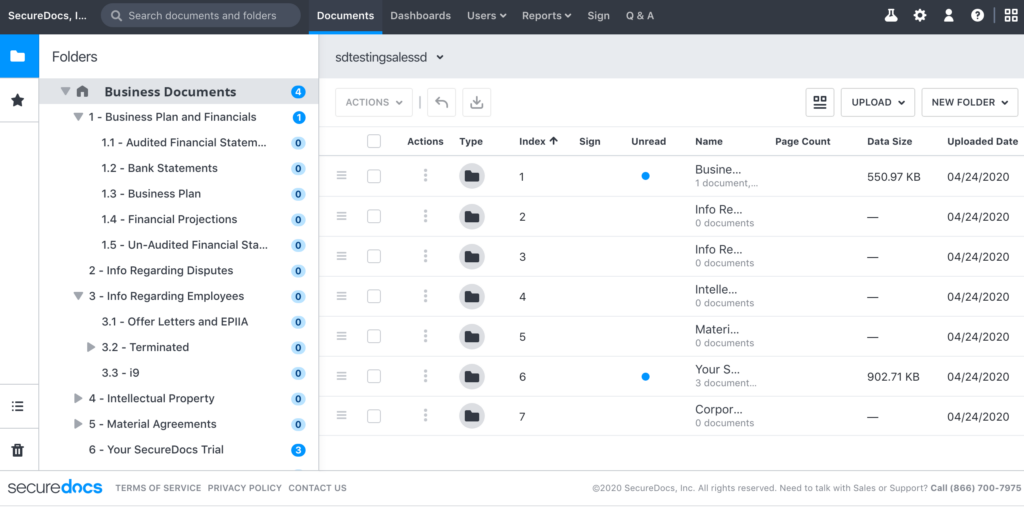
Ideals
Ideals offers a comprehensive VDR solution with advanced features and customizable user permissions for secure data sharing. Specifically for life sciences, Ideals offers:
- Customer success team providing support in 15 languages, ensuring effective communication and assistance for global life science projects.
- 10 data centers worldwide facilitating compliance with regulations requiring local data storage, which ensures reliable access to critical life science data.
- 8 customizable access levels to permit or deny viewing, printing, or downloading of files, ensuring strict control over sensitive healthcare data allowed only to authorized users.
- Limited time frameworks for document availability and the ability to revoke access remotely, which ensures data control and confidentiality, even after files have been downloaded.
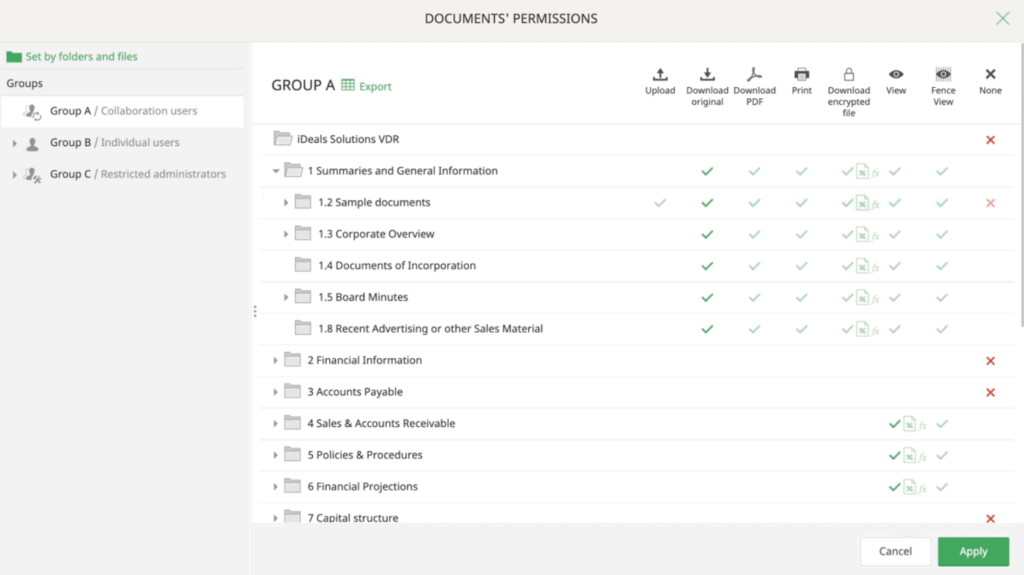
Intralinks
Intralinks is a trusted name in the VDR industry, known for its secure document-sharing capabilities and sophisticated access controls. Biotech firms benefit from the following features:
- A platform specifically designed to handle the diverse and intricate nature of life sciences projects.
- Advanced features like AI Redaction to maintain the confidentiality of sensitive data.
- Industry-leading security protocols that safeguard your vital documents.
- Access a dedicated global service team boasting over 20 years of experience in the life sciences sector.
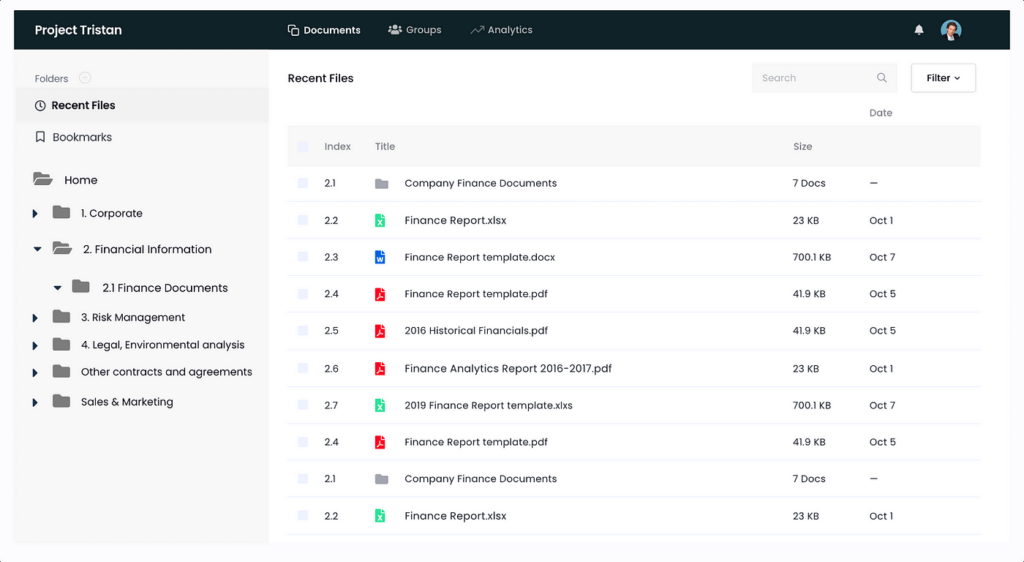
SecureDocs
SecureDocs also specializes in providing secure document management solutions tailored for biotech firms. In particular, it offers:
- Customizable dashboards for providing quick access to critical documents and insights.
- Granular permission settings to ensure compliance with regulatory requirements and protect confidential information.
- E-signatures with built-in signature capabilities to expedite approval workflows.
- Q&A for collaboration and communication among stakeholders and allowing for seamless exchange of inquiries and responses within the data room.
- Advanced search for quickly locating specific documents or information within a data room, which both saves money and improves productivity.
Summary
Now that you know what a data room for clinical trials is and what use cases it helps with, let’s briefly wrap up key insights from the article:
- Life science firms face significant challenges in commercializing their assets, from data organization to informed decision-making.
- Virtual data rooms serve as secure repositories for information of a sensitive nature, offering advanced security features like encryption and granular data room user access controls.
- In clinical trials, M&A due diligence process, fundraising, IP management, licensing/partnering, and IPO processes, virtual data rooms play crucial roles.
- Top VDRs for life sciences include Ideals, Intralinks, and SecureDocs, offering essential features tailored specifically to the needs of the life sciences industry.
Looking for more information on virtual data rooms for life sciences? Check out our home page, where you’ll find an insightful data room comparison and selection guide.